Fundamentals of Drug Therapy in Neurology
fundamentals of drug therapy in neurology provides a readable synopsis of how the classic pharmacology concepts such as pharmacodynamics and pharmacokinetics are related to therapy in neurology. This section also discusses the pathogenesis of potentially serious side effects such as skin rashes and liver disorders, and the role of pharmacogenetics in both drug effect and side effects.
Overview
Physical therapists and occupational therapists are non-prescribing healthcare practitioners. You may be asking yourself, "Why do I need to know this?" If that is your mindset, I hope that we can help you to see why it's important that you become competent with basic pharmacological principles. In all 50 states, physical therapists have autonomous practice ability. Some states won't allow long-term treatment without a physician referral, but they will allow evaluation. With autonomous practice, you have a professional responsibility to screen your patients' medications and be able to point out potential issues with those medications.
Additionally, medications significantly influence your patients' ability to participate in physical therapy. When I was beginning in home health, I had been a stay-at-home mom for several years. I was working in a patient's home, and I called their physician because I was concerned that my patient had a very low heart rate. It turns out the patient was on beta blockers and I had no idea that was why their heart rate had decreased. That's one of the reasons why I decided to become a champion for physical therapists to be able to understand and implement knowledge of medication into their practice.
Medicines can significantly influence a patient's ability to participate in therapy. Conversely, therapy can significantly influence the action of certain medications. Sometimes, therapy can hasten medication metabolism. Sometimes it can slow it. Sometimes it can impact how it is metabolized. As therapists, we need to be aware of that. If we have knowledge in that area, we can optimize both the pharmacological intervention and the physical therapy intervention.
First, I will provide a brief overview of pharmacology. Next, we will discuss the most common ways in which medications are used for central nervous system disorders. We will take a look at some case examples of patient problems to apply our knowledge and discuss as a group. I will try to point out some commonalities among diagnoses and medications. Finally, I will show you how to find reliable websites that provide accurate drug information.
Pharmacology Review
Pharmacology is the study of medications. Pharmacology can be divided into two subcategories: pharmacotherapeutics and toxicology. Pharmacotherapeutics is the study of the beneficial aspects of medication. Toxicology is the study of the harmful aspects of medication, those which may even lead to death. For the purposes of today's presentation, we will solely be looking at pharmacotherapeutics, which can be divided further into two more categories: pharmacokinetics and pharmacodynamics.
Pharmacokinetics is the study of what the body does to the drug. The drug is administered, and then it is absorbed into the bloodstream. It's distributed into certain systems within the body. It is metabolized and eventually excreted. The body is trying to get rid of the drug from the moment it's administered.
Pharmacodynamics is the study of what the drug does to the body. The drug exerts an effect on the cellular level, and the combined cellular level effects cause a systemic effect that can exert an influence throughout the body.
The primary mechanism of action is the means by which a drug produces an alteration in function. That usually occurs at a cellular level, with some type of interaction with a cellular receptor. The drug binds to a cellular receptor and causes a biochemical reaction that alters the cell function. If you remember back to physiology class, we have a lipophilic bipolar cell membrane, and embedded in the membrane are glycoproteins. The glycoproteins have communication with the extracellular space and also within the cell. Often, neurotransmitters will bind to these receptors and they cause some change. The drug can also bind to the receptor to cause some action.
To reiterate and summarize pharmacotherapeutics, it is helpful to look at the following flowchart. On the upper half of Figure 1, we can see the process of pharmacokinetics: what the body does to the drug. First, a dose of a drug is administered either enterally (i.e., by mouth) or parenterally (i.e., going around the digestive system). Parenteral administration is given through an IV or via some intramuscular or subcutaneous method. After administration, the drug is absorbed and eventually makes its way into the bloodstream. After absorption, we want the medication to be distributed to particular tissues so that it can have some pharmacological effect. As this distribution is going on, the drug is also being metabolized and excreted. This administration, absorption, distribution, metabolism, and elimination is all part of pharmacokinetics.
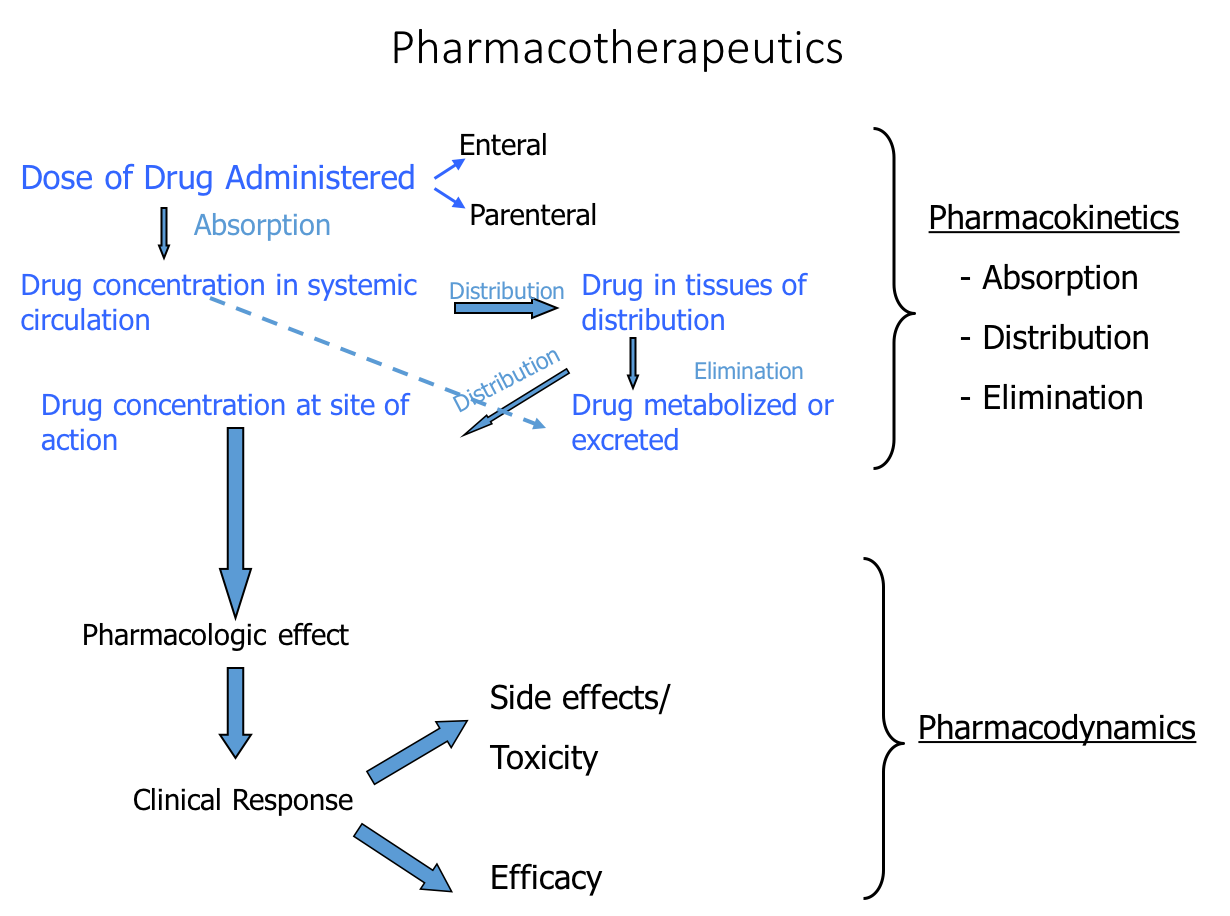
Figure 1. Processes of pharmacokinetics and pharmacodynamics.
The bottom portion of Figure 1 shows the process of pharmacodynamics: what the drug does to the body. When the drug reaches the site of action in the body in a sufficient concentration, it should have a pharmacological effect and provide some clinical response. For example, if I administered a medication for high blood pressure, the clinical response would be lowered blood pressure. Such a response as lowered blood pressure would be an efficacious response to that medication. Hopefully, our medications have a high level of efficacy and give the desired response, but medications are known to also give side effects and potentially toxic effects. That entire process is pharmacodynamics.
There are many different drugs for neurological disorders. I've tried to provide a structure for you to look at these drugs. If you encounter an unfamiliar drug, you can refer back to this structure, so you can compare and contrast the new drug with the drugs that you're familiar with.
Drugs are commonly given for central nervous system disorders for one of four purposes:
- To minimize secondary damage acutely, immediately after a traumatic event, such as:
- Spinal cord injury (SCI)
- Ischemic cerebrovascular accident (CVA)
- Traumatic brain injury (TBI)
- To manipulate neurotransmission (either to increase or decrease neurotransmission) in cases such as:
- Parkinson's disease (PD)
- Alzheimer's disease (AD)
- Psychiatric diseases
- To try to slow the disease progression, in cases such as:
- Multiple sclerosis (MS)
- Amyotrophic lateral sclerosis (ALS)
- To minimize signs/symptoms and secondary problems that may develop with neurological disorders:
- Hypertone/spasticity
Minimize Secondary Damage Following Acute Event
The central nervous system is composed of the brain and the spinal cord. Everything else, including the autonomic nervous system, is considered part of the peripheral nervous system. An acute event, usually traumatic, can occur in the central nervous system, the most common of which is an ischemic cerebrovascular accident. The next most common type is a traumatic brain injury, followed by traumatic spinal cord injury.
An acute event occurs that causes damage somewhere in the central nervous system. The damage is not ongoing. It's a static event that causes primary cell death and some secondary damage, but it's not progressive. You could contrast that to Parkinson's disease, which is a progressive disease (i.e., the damage keeps occurring). The central nervous system disorders that occur following an acute event usually are more of a static lesion. It occurs once; it's not going to keep occurring over time.
After a central nervous system injury, there is a series of events that occur (Figure 2). If a person receives a blow to the head, for example, that will result in a central core of cell death. That person will suffer primary cell death. In addition to that primary cell death, those neurons that are in communication with those now dead cells are very susceptible to secondary injury. The areas adjacent to initial injury that may die secondarily is called the penumbra. In fact, any cell that communicates with that core area is susceptible to later cell death. Any cell, whether it's in the other hemisphere of the brain or if it's further down in the spinal cord, is susceptible to cell death. Those interconnected areas that may eventually die are called the diaschisis.
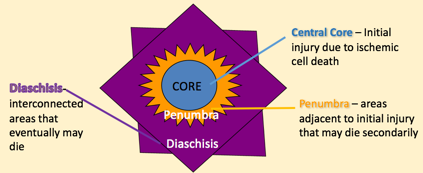
Figure 2. Events after a CNS injury.
Even if the initial area of core cell death is relatively small, that area grows over time. The penumbra is the area that's adjacent to the initial injury; because of the interconnectivity in the central nervous system, a significant lesion may grow from the initial central core death. This is not only true in the brain, but also true in a spinal cord injury. A traumatic injury may cause a central core, but over time (hours or days), that lesion could grow significantly.
The traumatic event causes an area where some cells are not getting the oxygen that they need. The trauma also causes the release of excitatory amino acids, which activate receptors that cause a major influx of calcium. All of this leads to excitotoxicity. A further cascade of events occurs that lead to inflammation in the area of injury, all of which leads to further cell death through necrotic pathways and apoptotic cell death, which causes an increase in the lesion's size. As the lesion size increases, it leads to an increase in the patient's functional deficits. You might start with a small lesion and end up with a very large lesion and a lot of functional deficits.
The pharmacological management after these central nervous system acute injuries is to try to decrease the excitotoxicity. If we can decrease this excitotoxicity and decrease the inflammation, we won't cause growth in the lesion and we'll have fewer functional deficits. That's the pharmacological premise in trying to give drugs that will help to decrease that secondary cell death.
Next, we will review some different diagnoses and take a look at some of the medications that are used for each diagnosis.
Acute Spinal Cord Injury
Research conducted in the 1990s by Bracken, et al in the New England Journal of Medicine looked at using a glucocorticoid (a steroid) in the acute management of spinal cord injury. They particularly looked at methylprednisolone (MP). This study showed such a significant improvement in those patients who were acutely given MP that they stopped the clinical trial, and this was implemented across the country as the standard procedure of giving a high dosage bolus IV of MP, followed by an infusion over the next 23 hours. It was found that this significantly decreased the inflammatory effects and decreased the secondary cell death. They also found that this must be given very early. The maximum window of opportunity is within the first eight hours after a spinal cord injury.
Acute Traumatic Brain Injury
Initially, glucocorticoids and MP were also considered for use with cases of acute TBI. Although it showed great promise in animal models, it led to a higher mortality rate in humans. The appropriate pharmacological mechanism to prevent secondary damage after acute TBI is a little bit different than that of acute SCI. After a TBI, the two main pharmacological goals are to maintain an optimal blood pressure and to normalize intracranial pressure. After a TBI, you don't want the blood pressure hypertensive (too high), but it's equally if not more important to not have the blood pressure get too low. If the blood pressure is too low, the brain is not getting the perfusion that it needs, leading to increased cell death. Research has shown that for every 10-point increase in systolic blood pressure, there is a decrease of almost 20% in the adjusted odds of death. It's very important to maintain normal blood pressure so that we can have appropriate perfusion in the brain. The most common medication used for that purpose is a sympathomimetic drug, phenylephrine HCL, which actually increases blood pressure. For those patients whose blood pressure is low, they will administer this medication. It increases peripheral vascular resistance in the periphery, but it does not do that in the blood vessels, and so we get better perfusion in the brain. It's administered intramuscularly or through IV.
With acute TBI, we also want to maintain normal intracranial pressure (ICP). Due to the nature of a TBI, an increase of intracranial pressure is very common, which can result in significantly further brain damage. The two agents that are most commonly used to control ICP are osmotic agents and barbiturates. Mannitol is the gold standard for normalizing ICP, keeping it at less than 15 mm Hg. Mannitol is an osmotic diuretic that has an effect on intracranial pressure. It enhances cerebral blood flow and brain metabolism. Initially, it can be a good medication to lower intracranial pressure. Use of Mannitol does require an intact blood-brain barrier. As such, it may not be appropriate to use with someone who has sustained significant head trauma. One of the side effects of Mannitol is pulmonary edema, which can later predispose the patient to pneumonia, hypotension and renal injury, as it is metabolized through the kidneys.
It should be noted that there has not been a large randomized controlled trial of Mannitol against a placebo because to do so would be to withhold appropriate treatment for patients with TBI.
Barbiturates are another common classification of medication used to lower the intracranial pressure, by altering the vascular tone. Barbiturates are a potent GABA facilitator. GABA is an inhibitory neurotransmitter. By inhibiting excitatory neurotransmission, barbiturates have the effect of suppressing brain activity. Suppression of brain activity will also lead to sedation, amnesia, and loss of consciousness. As such, barbiturates are used to initiate a medically induced coma. There are two medications (Pentobarbital and Secobarbital) that are frequently used for this purpose. Pentobarbital is short-acting and Secobarbital is medium-acting. Once these barbiturates do get into the system, it takes quite a while for them to be cleared, and for a patient to wake up from a medically induced coma.
Acute Ischemic Stroke
First, I want to differentiate between an ischemic stroke and a hemorrhagic stroke. An ischemic stroke occurs as a result of an obstruction within a blood vessel supplying blood to the brain. If no blood can get through, there is a hypoxic insult and some brain tissue dies. With a hemorrhagic stroke, a weakened blood vessel ruptures, resulting in hypoperfusion. Blood spills into or around the brain and creates swelling and pressure, damaging cells and tissue in the brain. The treatment for ischemic stroke is very different from hemorrhagic stroke. In fact, treatment for an ischemic stroke, if given to someone who is having a hemorrhagic stroke, will usually be fatal. The first step in the emergency room is to differentiate whether the patient is having an ischemic stroke or a hemorrhagic stroke.
For an ischemic stroke, blood pressure control is very similar to TBI, except that patients with an ischemic stroke often present with hypertension and not low blood pressure. Secondarily, they want to break down any clot that is causing the blood vessel occlusion using fibrinolytic drugs, and then give anticoagulants to prevent further damage. By doing that, that will help us to save and prevent secondary damage and save that penumbra and the diaschisis, so the patient has minimal or no functional deficits.
Fibrinolytic agents (commonly known as "clot busters") need to be administered within three hours of symptom onset. There are two fibrinolytic agents that are used frequently: streptokinase and tissue plasminogen activator (tPA). Tissue plasminogen activator is the more expensive of the two drugs. Streptokinase is a lot less expensive, however, it has a shorter shelf life. Hospitals have found that if streptokinase sits on their shelf, it expires and has to be discarded. tPA has a longer shelf life. Even though it's more expensive, they don't need to waste it as often. For that reason, more facilities use tPA than use streptokinase. The mechanism of action of both of these drugs is to activate plasminogen bound to fibrin. It breaks up a clot, which is what's usually causing the occlusion in the blood vessel. The fibrinolytic agents can be given through an IV. They also have been given directly in the brain, which requires a specialized facility.
The second line of defense to minimize secondary damage is to prevent further clots from forming, and that can be done through the use of anticoagulants. One of the most common anticoagulants administered parenterally (through an IV) is heparin. The most common drugs administered subcutaneously are Lovenox and Arixtra. Orally, the most common anticoagulant drug used is a vitamin K antagonist known generically as warfarin, or under the brand name, Coumadin.
Rehab Implications
Medications that are given acutely to prevent secondary damage do have some rehab implications. Acutely, you probably won't be working with a patient until they're deemed medically stable. If you are a working with a patient who may not be medically stable, you likely will be doing some passive, dependent activities (e.g., turning, maintaining mobility, working with respiration, preventing skin breakdowns).
The appropriate, effective, and safe utilization of drugs in the treatment of disease requires a basic understanding of the dose–effect relationships of medications.
Relating dose to effect requires a combined appreciation of pharmacodynamic
concentration–effect relationships, or what drugs do to the body, and of pharmacokinetic dose–concentration relationships, or what the body does to or with drugs.
For this reason, considerable attention is devoted to both the pharmacodynamic and
pharmacokinetic aspects of drug therapies. The aim of this chapter is to provide a
theoretical rationale that is necessary for appropriately interpreting the results of
basic and clinical neuropharmacology studies and for understanding many of the
drug treatment strategies commonly encountered in clinical neurology and
psychiatry.
Approximately one-fourth of all drugs prescribed worldwide exert their therapeutic actions on CNS targets. Of the top five selling drugs in this category, three
are antidepressants and two are atypical antipsychotics (1). The relative success of
pharmacological intervention is highlighted further when one considers that these
neurological disorders continues to expand steadily and the aim of this book is to provide a perspective for neurologists, psychiatrists, and other clinicians on how they can be used effectively. This perspective includes information about the pathophysiology of disease as well as drug interactions with the nervous system and the rest of the body. This introductory chapter deals with the fundamental concepts that are common to more specific treatment issues covered in detail in the rest of the book (Table 1–1). First we discuss some major molecular targets of drug action in the nervous system, an area that has expanded greatly as a result of the use of molecular genetic and electrophysiological techniques. The interaction of drugs with these targets is generally known as pharmacodynamics (PD) or the study of mechanisms of action and the relationship between drug concentration and effect. Then we discuss the absorption, distribution, metabolism and elimination of drugs, which determine their pharmacokinetics (PK). Taken together, a drug’s PD and PK characteristics define its therapeutic window or the range of dose and/ or blood level at which it has optimal effect with minimal side effects. For many drugs these characteristics are under genetic control and this is the major focus of the field of
note
pharmacogenetics. Genetic variation in the distribution and metabolism of drugs as well as in targets of drug action such as ion channels and neurotransmitter receptors are being recognized at an increasing rate. Pharmacogenetic factors can influence the dose of a drug required for treatment of individual patients, as well as its efficacy if a mutation is present in its molecular target of action. Another important factor that strongly influences drug therapy in neurology is the toxicity of these drugs. Because they target the nervous system, these drugs are prone to produce potentially disabling effects on cognition and other brain functions and this often influences the choice of drugs for individual patients. Finally we discuss the influence of ageon effects of nervous system drugs, especially on fetuses, pregnant women, and elderly individuals.
CLASSIFICATION OF DRUGS ON THE BASIS
OF THE RESPONSES THEY PRODUCE ON THEIR RECEPTORS
When a drug reversibly binds to the orthosteric (primary) site on its receptor one
of four outcomes are to be expected: the receptor becomes activated, the receptor
becomes partly activated, the receptor becomes inactivated, or the receptor is unable
to be activated. Consequently, drugs are generally classified based on their actions.
A drug is an agonist if it fully activates receptors, a partial agonist if it partly activates receptors, an inverse agonist if it inactivates receptors (and prevents them
from being activated), or an antagonist if it only prevents receptors from being
activated. For instance, the endogenous neurotransmitter DA is an (full) agonist of
DA receptors, and the antiparkinsonian drug bromocriptine is a partial agonist at
D2 receptors. Antipsychotics like haloperidol and clozapine may be inverse agonists of D2 DA receptors (4,5), whereas L-741,626 is an (neutral) antagonist.
Receptor activation is a thermodynamic process, whereby agonist binding
induces a conformational change in the receptor and converts it from the inactivated state (agonist low-affinity binding state) to the activated state (agonist highaffinity binding state). Typically, neutral antagonist binding is indifferent to the
conformational (affinity) state of its receptor, because it must only occupy the
orthosteric site rather than occupy and then induce a change in it. However, an
inverse agonist is a special type of antagonist in that when it binds to a receptor in
the activated state it converts it to the inactivated state. For this reason, inverse
agonists reduce the basal levels of constitutive receptor activity, which corresponds
to the (typically small) proportion of receptors that are in the activated state in the
absence of agonist. Such distinctions in the molecular mechanisms of action of
antipsychotic drugs that act on D2-like dopamine and 5-hydroxytryptamine (5-HT)2-
like serotonin receptors may be critical to understanding their unique therapeutic
profiles (4,6).
Although most drugs bind directly to the orthosteric site of the receptor, other
drugs bind at another (secondary) receptor site, called an allosteric site. Ligands
that bind to the allosteric site are known as allosteric modulators, because they
indirectly modulate the binding of primary ligands to the orthosteric site by
remotely altering the orthosteric-binding site. The modulation is said to be positive
if the modulator facilitates a primary ligand’s interaction with the primary site or
negative if the modulator attenuates its interaction with the primary site. The extent
to which the allosteric site and the orthosteric site are coupled, or their cooperativity,
can be weak or strong. Noncompetitive interactions, which result in a complete
occlusion of the orthosteric site leading only to a decrease in the maximum density
of sites with no change in affinity, are also allosteric in nature but they are a special
case of neutral cooperativity. Within the DA receptor family, for example, a diverse
Central Nervous System (CNS) Depressants and Stimulants
Prescription Drugs and Pain Medications: Part 2 of 3
Central Nervous System (CNS) Depressants
CNS depressants slow normal brain function. In higher doses, some CNS depressants can become general anesthetics. Tranquilizers and sedatives are examples of CNS depressants. CNS depressants can be divided into two groups, based on their chemistry and pharmacology: Barbiturates, such as mephobarbital (Mebaral) and pentobarbitalsodium (Nembutal), which are used to treat anxiety, tension, and sleep disorders.
Barbiturates, such as mephobarbital (Mebaral) and pentobarbitalsodium (Nembutal), which are used to treat anxiety, tension, and sleep disorders.
CNS depressants slow normal brain function. In higher doses, some CNS depressants can become general anesthetics. Tranquilizers and sedatives are examples of CNS depressants. CNS depressants can be divided into two groups, based on their chemistry and pharmacology:
 Barbiturates, such as mephobarbital (Mebaral) and pentobarbitalsodium (Nembutal), which are used to treat anxiety, tension, and sleep disorders.
Barbiturates, such as mephobarbital (Mebaral) and pentobarbitalsodium (Nembutal), which are used to treat anxiety, tension, and sleep disorders.
Benzodiazepines, such as diazepam (Valium), chlordiazepoxide HCl (Librium), and alprazolam (Xanax), which can be prescribed to treat anxiety, acute stress reactions, and panic attacks. Benzodiazepines that have a more sedating effect, such as estazolam (ProSom), can be prescribed for short-term treatment of sleep disorders.
There are many CNS depressants, and most act on the brain similarly—they affect the neurotransmitter gamma-aminobutyric acid (GABA). Neurotransmitters are brain chemicals that facilitate communication between brain cells. GABA works by decreasing brain activity. Although different classes of CNS depressants work in unique ways, ultimately it is their ability to increase GABA activity that produces a drowsy or calming effect. Despite these beneficial effects for people suffering from anxiety or sleep disorders, barbiturates and benzodiazepines can be addictive and should be used only as prescribed.
CNS depressants should not be combined with any medication or substance that causes sleepiness, including prescription pain medicines, certain over-the-counter cold and allergy medications, or alcohol. If combined, they can slow breathing, or slow both the heart and respiration, which can be fatal.
Discontinuing prolonged use of high doses of CNS depressants can lead to withdrawal. Because they work by slowing the brain’s activity, a potential consequence of abuse is that when one stops taking a CNS depressant, the brain’s activity can rebound to the point that seizures can occur. Someone thinking about ending their use of a CNS depressant, or who has stopped and is suffering withdrawal, should speak with a physician and seek medical treatment.
In addition to medical supervision, counseling in an in-patient or out-patient setting can help people who are overcoming addiction to CNS depressants. For example, cognitive-behavioral therapy has been used successfully to help individuals in treatment for abuse of benzodiazepines. This type of therapy focuses on modifying a patient’s thinking, expectations, and behaviors while simultaneously increasing their skills for coping with various life stressors.
Often the abuse of CNS depressants occurs in conjunction with the abuse of another substance or drug, such as alcohol or cocaine. In these cases of polydrug abuse, the treatment approach should address the multiple addictions.
Stimulants
Stimulants increase alertness, attention, and energy, which are accompanied by increases in blood pressure, heart rate, and respiration.
Stimulants increase alertness, attention, and energy, which are accompanied by increases in blood pressure, heart rate, and respiration.
Historically, stimulants were used to treat asthma and other respiratory problems, obesity, neurological disorders, and a variety of other ailments. As their potential for abuse and addiction became apparent, the use of stimulants began to wane. Now, stimulants are prescribed for treating only a few health conditions, including narcolepsy, attention-deficit hyperactivity disorder (ADHD), and depression that has not responded to other treatments. Stimulants may also be used for short-term treatment of obesity and for patients with asthma.
Stimulants such as dextroamphetamine (Dexedrine) and methylphenidate (Ritalin) have chemical structures that are similar to key brain neurotransmitters called monoamines, which include norepinephrine and dopamine. Stimulants increase the levels of these chemicals in the brain and body. This, in turn, increases blood pressure and heart rate, constricts blood vessels, increases blood glucose, and opens up the pathways of the respiratory system. In addition, the increase in dopamine is associated with a sense of euphoria that can accompany the use of stimulants.
Research indicates that people with ADHD do not become addicted to stimulant medications, such as Ritalin, when taken in the form and dosage prescribed.1 However, when misused, stimulants can be addictive.
The consequences of stimulant abuse can be extremely dangerous. Taking high doses of a stimulant can result in an irregular heartbeat, dangerously high body temperatures, and/or the potential for cardiovascular failure or seizures. Taking high doses of some stimulants repeatedly over a short period of time can lead to hostility or feelings of paranoia in some individuals.
Stimulants should not be mixed with antidepressants or over-the-counter cold medicines containing decongestants. Antidepressants may enhance the effects of a stimulant, and stimulants in combination with decongestants may cause blood pressure to become dangerously high or lead to irregular heart rhythms.
Treatment of addiction to prescription stimulants, such as methylphenidate and amphetamines, is based on behavioral therapies proven effective for treating cocaine or methamphetamine addiction. At this time, there are no proven medications for the treatment of stimulant addiction. Antidepressants, however, may be used to manage the symptoms of depression that can accompany early abstinence from stimulants.
Depending on the patient’s situation, the first step in treating prescription stimulant addiction may be to slowly decrease the drug’s dose and attempt to treat withdrawal symptoms. This process of detoxification could then be followed with one of many behavioral therapies. Contingency management, for example, improves treatment outcomes by enabling patients to earn vouchers for drug-free urine tests; the vouchers can be exchanged for items that promote healthy living. Cognitive-behavioral therapies, which teach patients skills to recognize risky situations, avoid drug use, and cope more effectively with problems, are proving beneficial. Recovery support groups may also be effective in conjunction with a behavioral therapy.
References:
1 Nora Volkow, et al., Dopamine Transporter Occupancies in the Human Brain Induced by Therapeutic Doses of Oral Methylphenidate, Am J Psychiatry 155:1325–1331, October 1998.
National Institute on Drug Abuse (NIDA)
National Institutes of Health (NIH)
U.S. Department of Health & Human Services
National Institutes of Health (NIH)
U.S. Department of Health & Human Services
For more information on addiction to prescription medications, visit http://www.drugabuse.gov/drugpages/prescription.html.
Drugs can be classified in many ways. For example, they can be classified according to:
- uses (medicinal or recreational)
- effect on the body (the specific effect on the central nervous system)
- source of the substance (synthetic or plant)
- legal status (legal/illegal)
- risk status (dangerous/safe).
The below sub-section summarises the major classifications of drugs including stimulants, depressants and hallucinogens. The group 'others' includes those psycho-active drugs that do not fit neatly in any other category. Some drugs can be classified in a number of categories, e.g. cannabis and ecstasy.
Classifying drugs by their effect on CNS
Stimulants
Tend to speed up the activity of a person's central nervous system (CNS) including the brain.These drugs often result in the user feeling more alert and more energetic.
Examples include:
- Amphetamines
- Cocaine
- Pseudoephidrine (found in medications such as Sudafed, Codral Cold and Flu)
- Nicotine
- CaffeineTop of page
Depressants (also known as relaxants)
Tend to slow down the activity of the CNS, which often results in the user feeling less pain, more relaxed and sleepy.These symptoms may be noticeable when a drug is taken in large amounts.
It is important to note that the term 'depressant' is used to describe the effect on the CNS, not mood.
CNS depressants are more likely to result in euphoria than depression, especially in moderate use.
Examples include:
- Alcohol
- Major tranquillisers
- Benzodiazepines (e.g. Valium, Temazepam) Opioids (heroin, morphine)
- Volatile substances (can also be classified as 'other' (glue, petrol, and paint).
Hallucinogens
Have the ability to alter a user's sensory perceptions by distorting the messages carried in the CNS. A common example is LSD (trips).Hallucinogens alter one's perceptions and states of consciousness.
Examples include:
- LSD
- Psilocybin (magic mushrooms)
- Mescaline (peyote cactus)
Other
Includes psycho-active drugs that do not fit neatly into one of the other categories, but which are clearly psycho-active, such as antidepressants (e.g. Zoloft) and mood stabilisers (e.g. Lithium).Examples include:
* Both ecstasy and cannabis can produce hallucinations, especially in cases of heavy use, or inexperienced users. However they are usually considered primarily as CNS stimulants and depressants respectively, as these effects are almost always present
Drugs can be named in a range of different ways from chemical formulae to street terms. The following demonstrates how the same drug can be named in different ways.
Cannabis (generic name) is also known as:
Cannabis (generic name) is also known as:
- Chemical name: Delta 9 - tetra hydro cannabinol (THC)
- Brand name: N/A
- Common term: Marijuana
- Street name: Pot/mull
- Chemical name: Ethanol
- Brand name: Victoria Bitter
- Common term: Beer
- Street name: Grog
- Chemical name: 7-chloro-1, 3-dihydro-1-methyl-5-phenyl-2H-1, 4-benzodiazepin-2-1
- Brand name: Normison
- Common term: Sedatives
- Street name: Pills
Exercises
Top of pageWorkplace learning/brainstorm acitivty
Question - What are street names in your local area for:- Cannabis
- Inhalants
- Benzodiazepines
- Ecstasy
- Amphetamines
- Hallucinogens
- Opioids
- Cocaine
Street names for drugs
Task - writing exercise
Write in any additional street names for each drug that is used in the area in which you work.Cannabis:
- Possible street names: Pot, grass, weed, reefer, joint, spliff, Mary-Jane, Acapulco Gold, rope, mull, cone, dope, skunk, bhang, ganja, hash, chronic, wacky tobacky
- Names in my area:
- Possible street names:
- Nitrous Oxide: laughing gas, whippits, nitrous
- Amyl Nitrate: snappers, poppers, pearlers, rushamies
- Butyl Nitrate: locker room, bolt, bullet, rush, climax, red gold
- Names in my area:
- Possible street names: Pills, downers, benzos, rohies, normies, vals, serries
- List names in my area:
- Possible street names: E, eccy, love drug, eggs, point, paste base, zip
- Names in my area:
- Speed, uppers, ice, crank, meth, crystal, whiz, snow, goee, shabu
- Names in my area:
- Possible street names:
- LSD: acid, trips, wedges, windowpane, blotter, microdot
- Psilocybin: mushies, blue meanies, magic mushrooms, gold tops
- PCP: angel dust, hog, loveboat
- Names in my area:
- Possible street names:
- Heroin: horse, hammer, H, dope, smack, junk, gear, boy
- Methadone: done ('doan')
- Names in my area:
- Possible street names:
- Cocaine: coke, flake, snow, happy dust, Charlie, gold dust, Cecil, C, freebase, toot, white girl, Scotty, white lady
- Crack: rock, base, sugar block
- Names in my area:
Drugs can also be classified in terms of their legal status. There are a variety of reasons why some recreational drugs are legal while others are illegal. The legal status of drugs is often due to historical and political factors rather than their harmful nature. For example, penalties are imposed for the use of some drugs, such as heroin, which only a small proportion of the population use, while others of apparently equal or greater danger are widely used, accepted and promoted, such as alcohol.
Formal sanctions such as laws that prohibit the use of certain substances can deter people from using those drugs. However, they do not necessarily stop use altogether. Prescriptions are another way of influencing the use and availability of drugs.
Formal sanctions such as laws that prohibit the use of certain substances can deter people from using those drugs. However, they do not necessarily stop use altogether. Prescriptions are another way of influencing the use and availability of drugs.
Task - writing exercise
Write the names of at least two or more psycho-active drugs in each category below.- Legally available to adults e.g. Over the counter painkillers
- Legal with prescription e.g. Ritalin
- Illegal to use e.g. Cocaine
Summary
- The most useful system of classifying drugs is by their effect on the central nervous system
- Stimulants speed up the CNS
- Depressants slow down the CNS
- Hallucinogens distort the message carried in the CNS
- Other - those drugs that do not easily fit into the other groups.
- Some drugs such as cannabis and ecstasy can fit into more than one category.
Distance learners
(A good point for student to contact facilitator.)Distance learners should take time now to reflect on their learning, check in with their facilitator and determine their progress
Drugs produce their effect on the body through two major processes. The first is the effect of the chemical properties of the drug on the central nervous system (CNS) which includes the brain and the spinal cord. This process is called pharmacodynamics. The second is how the drugs enter, are metabolised, and absorbed by the body. This process is known as pharmacokinetics. These two processes work together to produce a certain effect.
Pharmacodynamic processes
Neurons
A psycho-active drug must find its way to the bloodstream to have an effect on the brain. Once the drug reaches the brain, it can lodge on to specific receptor sites on the neurons which are sensitive to particular types of drugs. Each drug affects specific neurons in a number of parts of the brain. There are 13 billion neurons or nerve cells in each person's brain.Neurotransmitters
Many drugs seem to imitate neurotransmitters, the natural chemicals that facilitate or inhibit the transfer of electrical impulses between neurons. For example, opiate drugs such as heroin are thought to exert their drug action by mimicking endorphins which are naturally occurring proteins that reduce pain.Drug action
Like neurotransmitters, drugs can speed up (CNS stimulants) or slow down (CNS depressants) the transfer of electro-chemical messages between neurons in the brain. Messages between neurons can also be distorted when hallucinogenic drugs are taken.Pleasure centre
In addition to affecting the transfer of messages between neurons, drugs appear to act directly on 'pleasure centres' in the brain, which may explain the euphoria experienced by users of many different types of drugs. It is believed that the effect on the pleasure centre is highly rewarding for many young people and is crucial to the development of drug dependence.Review Quiz
Top of pageTask - writing exercise
Complete the following quick quiz which reflects your learning so far.- Pharmacology is :
- a branch of science that deals with emotions
- the study of how drugs work
- the study of living things
- Circle the drug that is not psycho-active.
- alcohol
- petrol
- antibiotic
- A neuron is a:
- chemical in the brain
- gap between nerve cells
- nerve cell
- Drugs work by:
- imitating neurotransmitters
- destroying brain cells
- creating dysfunctional neural pathways
- The euphoria (good feeling) that drug use promote is caused by:
- the distortion of electrochemical messages between neurons
- stimulation of pleasure centres in the brain
- elimination of withdrawal symptoms
- Drugs can be classified by their effect on the CNS. What are the three major groups called? Provide two examples of drugs that fit in each of these categories.
- Group 1
- Group Name -
- Example 1 -
- Example 2 -
- Group 2
- Group name -
- Example 1 -
- Example 2 -
- Group 3
- Group name -
- Example 1 -
- Example 2 -
- Group 1
- Why is it necessary to have a fourth group called 'others'?
I am very glad I came across this post and stopped to read it from beginning to end, as it left on a very positive note. I was in the beginning of my own journey with MS and the depression it's was giving me was unbearable , I found some encouragement from several blogs and last year in seeing Rochelle make her personal goals after overcoming the disease with natural medicine I have to tried it also .I’ve kind of resigned to the fact that this is how life will be for me back until I found herbs that stop this multiple sclerosis easily and relief all the Fatigue and other symptoms I was experiencing ,I’m passing this info to anyone at there because ww w .multivitamincare .org has the right cure and caregiver to this disease ….I took various supplements, medicine prescribed by neurologist,massage and physiotherapy still the disease is was progressing very fast until the the MS formula from that caregiver .
ReplyDeleteMy husband was diagnosed with early onset Parkinson's disease at 57.his symptoms were shuffling of feet,slurred speech, low volume speech, degradation of hand writing, horrible driving skills, right arm held at 45 degree angle, things were tough for me, but now he finally free from the disease with the help of total cure ultimate health home, he now walks properly and all symptoms has reversed, he had trouble with balance especially at night, getting into the shower and exiting it is difficult,getting into bed is also another thing he finds impossible.we had to find a better solution for his condition which has really helped him a lot,the biggest helped we had was ultimatehealthhome they walked us through the proper steps,am highly recommended this ultimatehealthhome@gmail.com to anyone who needs help.
ReplyDeleteMy husband was diagnosed with early onset Parkinson's disease at 57.his symptoms were shuffling of feet,slurred speech, low volume speech, degradation of hand writing, horrible driving skills, right arm held at 45 degree angle, things were tough for me, but now he finally free from the disease with the help of total cure ultimate health home, he now walks properly and all symptoms has reversed, he had trouble with balance especially at night, getting into the shower and exiting it is difficult,getting into bed is also another thing he finds impossible.we had to find a better solution for his condition which has really helped him a lot,the biggest helped we had was ultimatehealthhome they walked us through the proper steps,am highly recommended this ultimatehealthhome@gmail.com to anyone who needs help.
ReplyDelete